Risk-Based Pharmacovigilance Auditing and Inspection Preparation
Life Science companies face an increasingly complex set of international regulations in their commitment to patient safety and Good Pharmacovigilance Practice (GVP). For evaluating the performance of the internal processes in a company the use of audits or inspections by external experts is common practice.
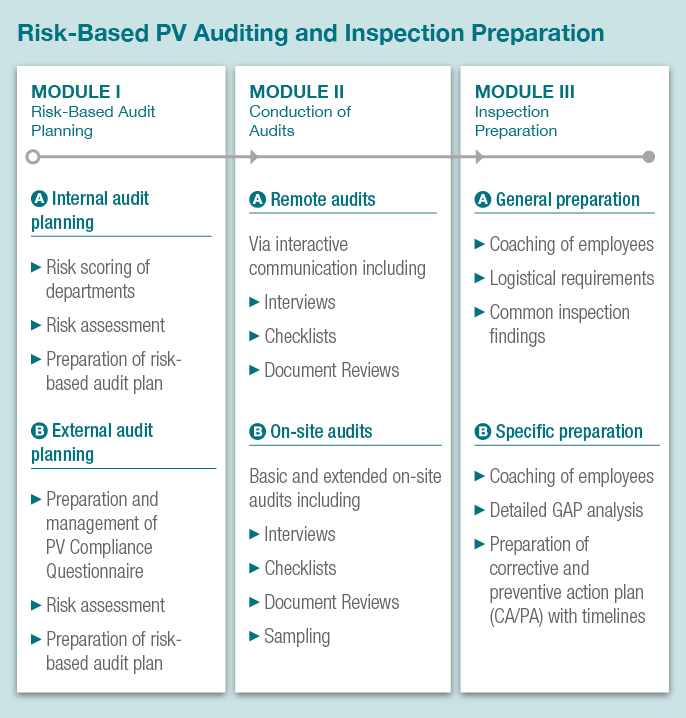
- Companies have to review periodically all components of their PV system and monitor compliance of contracted partners with PV duties set out in contractual agreements. In order to keep pace with this requirement MAHs are encouraged to use risk-based audits of their PV system and of its quality system.
- Dates and results of these audits have to be documented and are presented in the Pharmacovigilance System Master File (PSMF).
- Our modular risk-based auditing concept offers a cost-effective review fully tailored to your individual needs and requirements by allowing you to select a single module or the whole system.
Our Concept
We perform and document risk-based audits of your PV system in line with GVP as well as with DIN EN ISO 19011 to guarantee compliance with current legal obligations.
Please do not hesitate to contact us for an individual offer.